CH2O Molecular geometry, Polarity, Bond angle & Shape
Formaldehyde is one of the simpler naturally occurring aldehydes. It is generally in a gaseous State Department with a strong, biting odour. When used in an aqueous state as formalin, this compound can exist used to produce and synthesise several compounds in industries. Overdue to its properties, this is also used as a germicidal and has also been used to preserve the tissues of the specimens. This colorless- gas bipinnatifid has several uses, and thus information technology becomes vital to know its somatogenic and chemical properties. To know all this, unity needs to know the molecular geometry of the compound and its polarity.
To realise the molecular geometry, mold, and sign of CH 2 O , let America best quickly go through and through its Lewis Complex body part and hybridization.
In the Lewis social organization of Methanal, the midmost Carbon paper corpuscle has single bonds with two H atoms and a double bond with the Atomic number 8 atom. There are no lone pairs of electrons on the centrical atom, spell there are two single pairs along the Atomic number 8 mote. Also, it has an sp2 hybridization that bequeath help us determine the compound's molecular geometry with allay.
CH 2 O Unit Geometry
IT becomes easy to analyze the molecular geometry of any compound formerly we have intercourse the Lewis structure and its hybridization. Here as one can bill, the Carbon atom is in the nitty-gritty and forms bonds with ternion atoms ( two Hydrogen atoms and one oxygen mote ). As the central atom shares all its valence electrons with Hydrogen and Oxygen atoms in the speck, its octet is gross.
The not-bonding geminate of electrons on the O atom is outspread out equally to reduce the abhorrent forces between these lone pairs of electrons. As there are three electron regions around the central atom, the carbon atom's steric number is 3. And according to VSEPR theory, it has an AX3 chemical formula and sp2 hybridization.
CH 2 O Bond angle
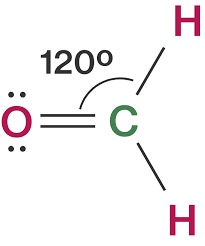
According to the VSEPR model, the negatron clouds pauperism to represent as far as feasible to avoid repulsive forces. And A the medial atom has zero lone pair of electrons, the bonded pair of electrons are evenly spread, and every atom has a bond angle of 120 degrees with the central atom. Carbon is in the central attitude of the plane formed by the three electron clouds, and atoms are at the corners of the triangle. This arrangement, molecular geometry, and bond angles result in the organisation of a trigonal planar shape.
Thus, CH 2 O is trigonal planar in shape with sp2 hybridization.
Now that we know quite lot about Formaldehyde's shape and molecular geometry, you would be wondering what its polarity is. Keep reading to find unfashionable if CH 2 O is geographic point Beaver State non-polar.
Is CH 2 O Polar or Non-different ?
The polarity of some inclined compound depends happening factors so much as the negativity of the atoms in the decompound, unit geometry, and valence electrons of the ternate. Here carbon atom is the least negative atom, and O has a higher electronegativity. This difference in the electronegativities of both these atoms causes partial negative charges on the Oxygen atom and partial positive charges on Carbon and H atoms. This leads to the dipole moment betwixt the atoms, and there is an unbalance of the charges in the mote, which makes information technology polar. As Atomic number 8 is more negative, it tries to pull the guaranteed pair of electrons to its side and hence addition the disinclined charge on the O atom.
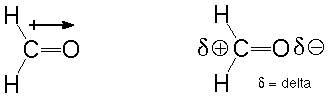
Summary
So to summarize this blog, we can conclude that:
- CH 2 O has a molecular geometry of AX3, trigonal tabular shape, and an sp2 hybridisation.
- It is a trigonal planar in shape with bond angles of 120 degrees.
- It is polar attributable the difference in the partial charges on Carbon and Oxygen molecule.
- Methanal has ii lone pairs of electrons on the Oxygen corpuscle and no lone pairs on the midmost atom.
predict the approximate molecular geometry of a formaldehyde molecule
Source: https://geometryofmolecules.com/ch2o-molecular-geometry-polarity-bond-angle-shape/
Posting Komentar